General Introduction of Organic Chemistry
General Introduction of Organic Chemistry: Overview
This topic covers concepts, such as, General Organic Chemistry, Urea : The First Synthetic Organic Compound, Wedge-and-dash representation of CH4 & Molecular Models of CH4 etc.
Important Questions on General Introduction of Organic Chemistry
Total number of and -bonds are in naphthalene is:
In which of the following molecules, all atoms are not coplanar?
Which one of the following does not have hybridised carbon ?
In the compound,  , the bond is of the type –
, the bond is of the type –
The hybridisation of carbon atoms in the C-C single bond of is:
Which of the following represent the given mode of hybridisation from left to right?
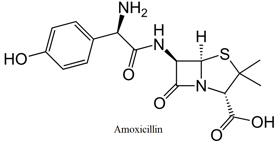
In the molecule given above how many atoms are unhybridised.
The ratio of and hybrid orbitals in the following molecule is
Write the maximum number of atoms having same hybridisation in the following structure
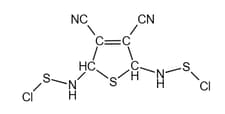
The carbon atoms in are
The molecule which contains and bond in it is
The number of and bonds in the compound
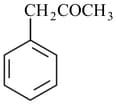 respectively are
respectively are
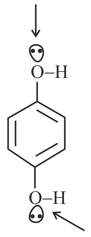
The hybridisation of the above oxygen atoms of -OH group is
Draw the complete formula of methane according to the structural model.
In Wedge-Dash notation for three-Dimensional representation of an organic molecule, the dashed bond represents.
In the ball-and-stick model, both the atoms and the _____ are shown in the molecule of methane.
The decreasing order of catenation among the Group- elements is
Arrange the following bonds in decreasing order of bond energy :
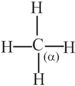
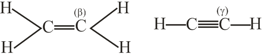
How many pi bonds and sigma bonds are present in following molecule?
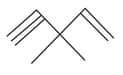
Give the structure of the following compound:
-Trichloroethane
